Understanding the Properties of Flexible Graphite
Flexible graphite is a material that is prominent in the industrial world, specifically due to its applications in heat management and fluid sealing. Experts understand the importance of flexible graphite in these respective fields because of its unique characteristics but the average person may not. The purpose of this article is to help the average person understand what flexible graphite is and what its properties are.
What is Flexible Graphite?
Flexible graphite, also known as graphite foil, expanded graphite or Grafoil®, is manufactured from expandable flake graphite. Graphite, which is pure carbon, has layers of carbon atoms which form strong hexagonal bonds. Each of such two dimensional layer is called a graphene sheet. Weaker bonds exist between each of the graphene sheet layers. Flexible graphite is created by submerging flake graphite into hydrofluoric and sulfuric acid. This weakens the bonds between each of the carbon layers, so the flake graphite becomes expandable. Then expose expandable graphite to intense heat, expanding the graphite, following press process to form the graphite into flexible graphite.
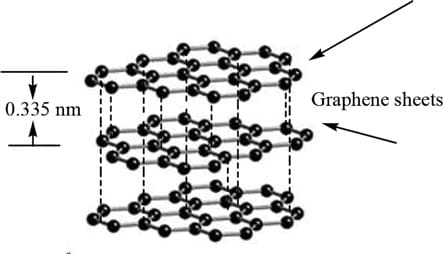
Graphite is non-metallic, formed by metamorphosis of organic-rich sedimentary rocks. It is the only naturally occurring non-metal that is a good electrical and thermal conductor. Due to its unique properties and conformable nature, flexible graphite is made into the following useful forms:
- Sheets and laminates (used to make gaskets)
- Rolls (used for gaskets, thermal liners, heat conductors, solid lubricant, electromagnetic interference (EMI) shielding)
- Tape and rings (used for valve-stem packing, gaskets, and seals)
- Packing (used in pump gland packing, valve stem packing, packing (seal) for mixers, agitators, joints)
Properties of Flexible Graphite
As with any structural material, flexible graphite has a long list of scientific specifications that can sound overwhelming to an interested layman. Terms such as bulk density, tensile strength, working temperature, and thermal conductivity are present in most if not all websites of various flexible graphite distributors. Let’s try to understand what these properties represent.
- Bulk density – Density is defined as the compactness of a substance. Mathematically it is mass per unit volume:

Bulk density is a term commonly used for quantifying compaction in soils, reflecting its structural support. It is also used to describe the compactness of flexible graphite. Bulk density considers the compactness of solid and pore space in a material, whereas density considers just solid material. It is an appropriate term for both soil and flexible graphite: soil due to its inhomogeneous natural; flexible graphite due to the “gaps” that are created when expandable flake graphite is treated with acids and exposed to intense heat. The higher the bulk density of an object, the more compact it is. For example, given the same size and shape, the bulk density of a brick will be higher than the bulk density of a sponge.
Bulk density for flexible graphite is given in pounds per cubic foot or grams per cubic centimeter.
- Tensile strength – Tensile strength is defined as the resistance of material breaking under tension. Tension is the act of something stretching or straining. Tensile strength is similarly defined as the stress at which an applied force causes the material to lengthen and break. The formula for stress is applied load per cross-sectional area:

Synonyms for tensile strength include durability, hardness, inflexibility, and toughness. There are many more. Tensile strength is vital in the field of mechanical engineering since it calculates the limits of strength for a given material. Below is a table of UTS (ultimate tensile strength) values of known materials:
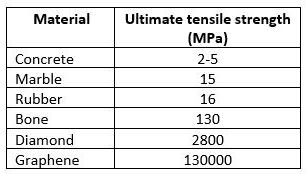
Source: “What is Ultimate Tensile Strength?” by Ashish Tiwari 11/28/2017; www.sciabc.us/jdw9G
Tensile strength is measured in psi (pounds per square inch) or MPa (megaPascals).
- Carbon content – Carbon content represents the purity of flexible graphite. The carbon content of various flexible graphite products range from 95% to 99.9%. Higher purity (higher carbon content) flexible graphite is used for nuclear applications. Lower purity flexible graphite is mostly used for industrial applications. Carbon content is given as percent carbon content.
- Sulfur content – Sulfur content represents the amount of sulfur contamination, in parts per million (ppm). Sulfur can exist in the raw flake graphite material used to make flexible graphite. A sulfur compound is added in the production process due to the use of sulfuric acid. Sulfur content is proportional to corrosion in metals, so it is important to minimize sulfur content in flexible graphite since flexible graphite is used with metals in both fluid sealing and heat management applications. Industrial grades of flexible graphite have maximum sulfur content level of 1000 ppm. Nuclear grades have a maximum sulfur content of ~600 ppm.
- Leachable chloride content – Leachable chloride content represents the amount of contamination from chloride contamination, in ppm. Leachable means to remove from a substance using percolating liquid. Chloride is a type of halide, and leachable halides are a primary concern due to its corrosive nature, especially with stainless steel. Most grades of flexible graphite have a maximum leachable chloride content of 50 ppm, which is acceptable for most industrial applications. Nuclear grades contain a leachable chloride content of no more than 20 ppm.
- Working temperature – Working temperature of a material is the temperature range at which that material maintains its structural integrity. Temperature maximums are commonly thought of at the mention of working temperature, but temperature minimums are important too. For example, ordinary carbon steel becomes brittle at temperatures well below 0 degrees C. The working temperature changes based on material type. Here are some examples of working temperature maximums for common materials:
Working temperature maximums for common materials
| Material | Maximum Working Temperature (⁰C) |
| Ceramics | 1000 |
| Glass | 500 |
| Wood | 150 |
| Rubber | 250 |
Environment is also a factor with working temperature of various materials. Three different environments are described when listing the working temperature range of flexible graphite:
Working temperature range of flexible graphite
| Environment | Minimum | Maximum |
| Oxidizing atmosphere (i.e. air) | -400 ⁰F (-240 ⁰C) | 950 ⁰F (510 ⁰C) |
| Mild oxidizing atmosphere (most gasket applications) | -400 ⁰F (-240 ⁰C) | 1500 ⁰F (850 ⁰C) |
| Non-oxidizing atmosphere | -400 ⁰F (-240 ⁰C) | 5400 ⁰F (3000 ⁰C) |
- Thermal conductivity – Thermal conductivity is a measure of a material’s ability to conduct heat. It is a parameter to consider when dealing with heat transfer. The equation for thermal conductivity is complicated, mainly because it is presented in many different forms. The equation for thermal conduction is:
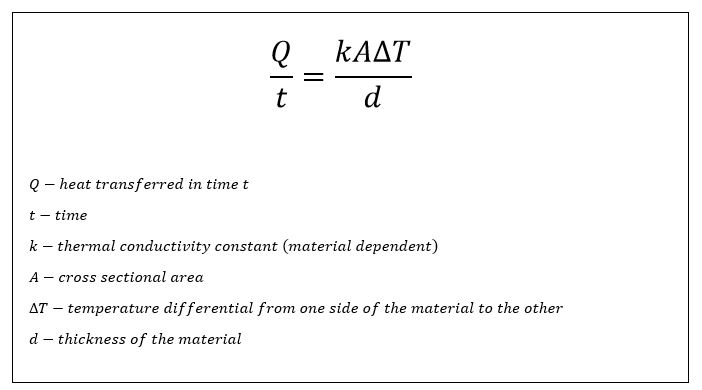
The left side of the equation (Q/t) represents joules of heat energy transferred through the given material per unit second. Joules per second is a watt. The thermal conductivity is represented by the constant k. Let’s look at a practical example. The thermal conductivity of wood is different from the thermal conductivity of copper. Heat transfers faster through copper than is does wood, therefore the thermal conductivity of copper is larger than the thermal conductivity of wood (similarly, k of copper is larger than k of wood). Two values are given for the thermal conductivity of flexible graphite:
- Parallel to sheet surface – The thermal conductivity for flexible graphite parallel to its surface is 140 watts per meter-kelvin (W/mK)
- Through thickness – The thermal conductivity for flexible graphite through its thickness is 5 W/mK.
Thermal conductivity of flexible graphite is said to be anisotropic. This means that flexible graphite’s thermal conductivity changes based on its direction. The thermal conductivity of flexible graphite is high along its outer surface but low when going through the material. This feature gives flexible graphite the dual functionality of being a good heat-sink in one direction and a good heat insulator in another. Units for thermal conductivity are given as W/mK or BTU-inches per hour-square feet-degrees Fahrenheit (BTU∙in/ft2∙h∙⁰F)
- Coefficient of friction – The coefficient of friction is the force due to friction between two objects. The equation for force due to friction is:
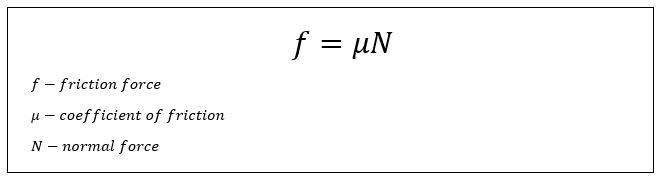
The coefficient of friction is a unit-less value that is based on two materials. Here are some examples:
Coefficient of friction (static) for various materials
| Materials | μ (static) |
| Steel on steel | 0.74 |
| Rubber on concrete | 1.0 |
| Ice on ice | 0.1 |
Notice that the coefficient of friction for materials that are “slippery” are low (i.e. ice on ice) compared to materials that are “stickier” (i.e. rubber on concrete). So if one wants to minimize friction they would seek two materials with a relatively low coefficient of friction.
Distributors of flexible graphite present coefficient of friction against steel at varying pressures. Mineral Seal Corporation (Minseal) gives the following:
- Coefficient of friction against steel: 0.018 @ 4 psi (0.03 Mpa)
0.157 @ 12 psi (0.08 Mpa)
Conclusion
Flexible graphite has become a very useful material in a variety of industries. Its unique characteristics are described with complicated scientific equations and concepts.
Most flexible graphite distributors list more properties than the eight mentioned in this article. These other properties (tensile strength, compression strength, compressibility, creep, etc…) will be covered in a later article.
Hopefully this article has helped the average person understand these properties a little better. And hopefully the average person has a stronger appreciation of flexible graphite and its many features.
