Is there a Relationship between Flexible Graphite and Graphene?
Flexible graphite and graphene are two carbon based materials relevant to many industries today. Although derived from the same atomic matter, these two materials contrast each other in a number of ways. Both are in a different stage of their timelines with regard to innovation. Both possess properties unique to each other. Flexible graphite is produced abundantly and used throughout several industries; while graphene, considered by the material science community to be the next major innovation, is still in its research phase. Besides being comprised completely of carbon atoms, are these materials related in any other way?
What are they and how are they made?
Flexible graphite and graphene are both made from graphite, a pure form of carbon, but what are they exactly? First, let’s talk about graphite. Graphite is an allotrope of carbon, meaning it is one of multiple forms carbon in the same physical state. Other allotropes of carbon include charcoal and diamond.

Diamond & graphite, allotropes of carbon 
Charcoal, another allotrope of carbon
The carbon atoms for each allotrope are bonded in a unique way. Graphite consists of layers of carbon atoms arranged in a 2-dimensional honeycomb pattern. Covalent bonds between atoms in the 2-dimensional honeycomb arrangement are very strong, while bonds between the layers are weak.
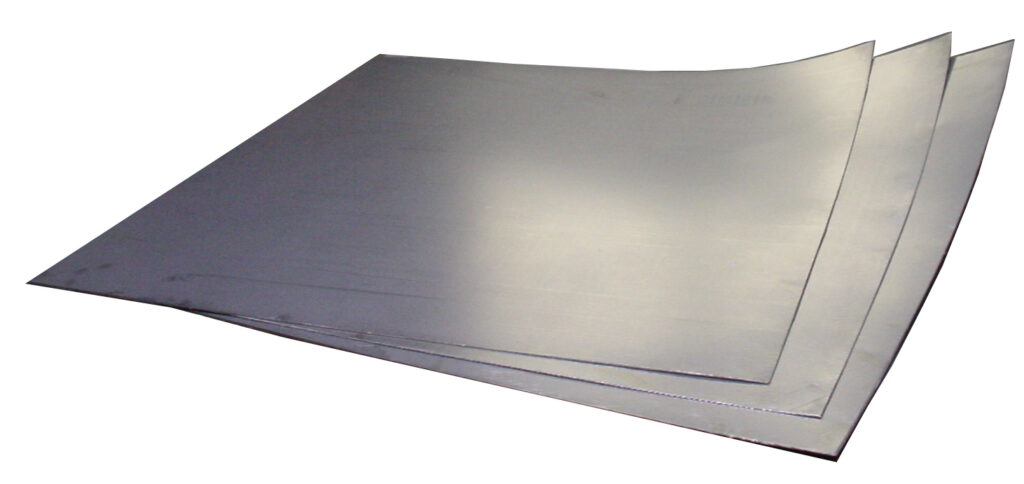
Flexible graphite is made from expandable flake graphite. Exposing flake graphite to chemicals and heat weakens the already weak bonds holding each 2-dimensional honeycomb layer of carbon atoms together, allowing for the graphite to expand. Flexible graphite is then constructed by compressing the expanded graphite into sheets.
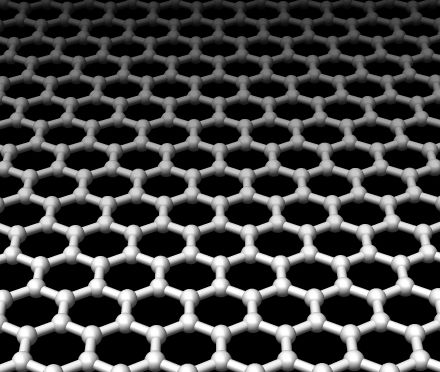
Graphene is made from graphite, sort of. It may be more accurate to say that graphene is extracted from graphite. Recall that graphite consists of multiple layers of 2-dimensional sheets of carbon atoms, with strong covalent bonds arranged in a hexagonal pattern. Graphene is simply one of these layers, which has the thickness of one carbon atom.
Graphene production is not yet fully realized. Multiple methods have been experimented with, including:
- mechanical exfoliation
- chemical vapor deposition
- crystal growth
- flash joule heating
Mechanical Exfoliation
The very first samples of graphene were produced in 2004 by Andre Geim and Kostya Novoselov at the University of Manchester. They did so by using sticky tape to remove layers from a chunk of graphite, over and over again. They repeated this process until they ended up with a layer one atom thick. This process (mechanical exfoliation) is still used today but cannot be used for industrial production.
Chemical Vapor Deposition
Chemical vapor deposition (CVD) is a process that uses chemical precursors in a heated vacuum chamber to deposit a thin film of solid material by condensation onto a chilled substrate. Details omitted, making graphene by CVD requires two steps:
- Formation of carbon from precursors
- Formation of graphene from carbon
Crystal Growth
It may be possible to grow graphene crystals from a carbon-rich source. Crystal growth is also often used in conjunction with CVD in order to produce graphene crystals .
Flash Joule Heating
Flash joule heating can be used to make graphene by placing a source of carbon (i.e. lots of different things, like used rubber tires) between two electrodes, applying short electrical pulses to rapidly raise the temperature to 3000 degrees C, which will then break apart all chemical bonds in the carbon containing material. The non-carbon elements will transform from directly from solid to gas (skipping the liquid phase), leaving the carbon elements to remain, which will in turn restructure into graphene.
How are they used?
Flexible graphite and graphene are in different stages of use and development. Flexible graphite is already an established, widely used product. Graphene is not commercially produce yet and is still being developed.
Flexible graphite is a prominent material choice for heat management and chemical resistance applications. It is a primary sealing material used in many industries today.
Graphene is one of the most intriguing materials to the scientific and industrial communities today because of its long list of properties . It is believed to be the hardest material ever tested, about 200 times stronger than steel and stronger than diamond. It is flexible and elastic, which potentially allows it to be shaped in many ways. Since graphene is only 1 carbon atom layer thick it is remarkably thin and light. Supposedly you can cover more than an entire soccer field with a sheet of graphene and it would weigh under a gram! It has very high thermal and electrical conductivity. Because graphene is transparent and an exceptional electrical conductor it has many applications in the field of optics. The list of properties for this amazing material doesn’t seem to end.
Is there a relationship?
Is there a relationship between flexible graphite and graphene? Obviously both can be derived from graphite. Both are geometrically versatile since they are flexible and can be shaped in a variety of ways. Both are thermally and electrically conductive.
Can one be used to make another? Recall that flexible graphite is created using chemical and heat treatments to weaken the bonds between each 2-dimensional layer in bulk flake graphite. If each layer is separated more in this process is it possible to make mechanical exfoliation of graphene easier? Chemical vapor deposition and crystal growth have been used together to grow graphene from chemical precursors. One major challenge with this method is to extract the newly formed graphene from the chilled substrate without causing damage. Can flexible graphite or some flexible graphite based composite be used instead of the typical copper substrate to make the extraction of graphene more efficient?
Unfortunately, the ideas mentioned above do not come with strong scientific backing but are merely a product of brainstorming. What is well known is that successful production of graphene on an industrial scale has become a top priority for the material science community.
